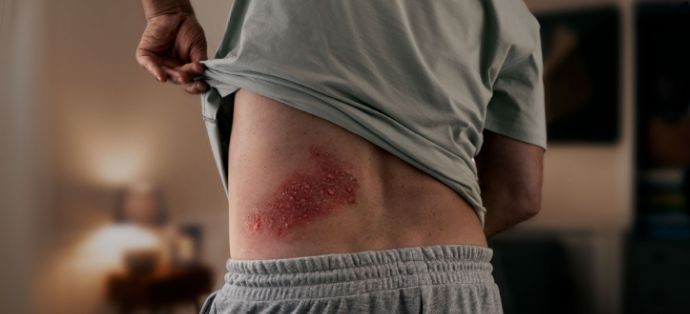
ARE YOUR PATIENTS AT INCREASED RISK?
AGE AND ADDITIONAL RISK FACTORS FOR SHINGLES
Patients ≥50 years old are at increased risk of shingles1
- 99.5% of people ≥50 years old are infected with the varicella zoster virus (VZV)1,2
- In 1 in 3 people, dormant VZV reactivates in their lifetime and causes shingles—a blistering rash that can be excruciatingly painful, typically lasting 7 to 10 days1,3
- The risk of developing shingles sharply increases starting at 50 years old—and continues to increase with age1


Data from a meta-analysis assessing risk factors for HZ. The analysis included a total study population of 198,751,846 individuals, with 3,768,691 HZ cases across 88 studies (68 cohort and 20 case-control studies) from 1966 to 2019. The populations in these studies ranged from people aged 3 months to 104 years old. Eighteen risk factors were identified in the meta-analysis, note not all are presented here. Limitations included the following: most studies were observational and had a higher likelihood of bias; the majority of studies used administrative data, which are subject to miscoding, errors, and can vary between practitioners; finally, heterogeneity was high across studies. This list is not exhaustive and may not present all conditions associated with an increased risk of HZ.4
Cardiovascular conditions included in each individual study in the meta-analysis varied by study and included heart disease, heart failure, hypertension, hyperlipidemia, stroke, atrial fibrillation/flutter, and other cardiovascular disease.4
SHINGRIX is a vaccine indicated for prevention of herpes zoster (shingles) in adults aged 50 years and older.
SHINGLES COULD HAVE MORE OF AN IMPACT THAN YOU REALIZE
In studies, ~9 in 10 patients with shingles experienced clinically significant pain.6,‡
- Patients were asked to rate their “worst pain” on a scale of 0 to 10 using the ZBPI. Clinically significant pain was defined as a score of 3 or greater6
Shingles can lead to serious and long-lasting complications.1
- PHN occurs in 10%-18% of individuals with shingles. This nerve pain lasts for months (≥90 days) and, in some cases, years1
SHINGRIX is not indicated for the prevention of PHN or other herpes zoster-related complications.5


Data from a post hoc analysis of 2 phase 3 trials of participants in the placebo groups with a confirmed case of herpes zoster in adults ≥50 years old (n=280) and ≥70 years old (n=240).6
UK observational study using Clinical Practice Research Datalink. Among 119,413 patients with shingles (median age 61 years) diagnosed between January 2000 and December 2011, 5.8% developed PHN (defined as pain persisting for ≥90 days following shingles diagnosis). Odds ratios for PHN were modeled for select comorbidities and adjusted for age, sex, socioeconomic status, HIV, leukemia, lymphoma, myeloma, hematopoietic stem cell transplantation, other unspecified cellular immune deficiencies, rheumatoid arthritis, systemic lupus erythematosus, inflammatory bowel disease, COPD, asthma, CKD, depression, personality disorder, diabetes, recent cancer diagnosis, smoking, BMI category, site of zoster, antivirals, and immunosuppressive therapies.7
VACCINATION STARTS WITH A STRONG RECOMMENDATION
Your strong recommendation can positively influence your patient’s vaccination decision.8



SHINGRIX is not indicated for the prevention of herpes zoster-related complications.5
See the strong recommendation discussion guide here.
aOR=adjusted odds ratio; BMI=body mass index; CI=confidence interval; CKD=chronic kidney disease; COPD=chronic obstructive pulmonary disease; HIV=human immunodeficiency virus; HZ=herpes zoster; PHN=postherpetic neuralgia; RR=relative risk; ZBPI=Zoster Brief Pain Inventory.
References: 1. Harpaz R, Ortega-Sanchez IR, Seward JF; Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2008;57(RR-5):1-30. 2. Kilgore PE, Kruszon-Moran D, Seward JF, et al. Varicella in Americans from NHANES III: implications for control through routine immunization. J Med Virol. 2003;70(suppl 1):S111-S118. 3. Shingles Symptoms and Complications. Centers for Disease Control and Prevention. April 19, 2024. Accessed March 18, 2024. https://www.cdc.gov/shingles/signs-symptoms/index.html 4. Marra F, Parhar K, Huang B, Vadlamudi N. Risk factors for herpes zoster infection: A meta-analysis. Open Forum Infect Dis. 2020;7(1):ofaa005. 5. Prescribing Information for SHINGRIX. 6. Curran D, Matthews S, Boutry C, Lecrenier N, Cunningham AL, Schmader K. Natural history of herpes zoster in the placebo groups of three randomized phase III clinical trials. Infect Dis Ther. 2022;11(6):2265-2277. 7. Forbes HJ, Bhaskaran K, Thomas SL, et al. Quantification of risk factors for postherpetic neuralgia in herpes zoster patients: A cohort study. Neurology. 2016;87(1):94-102. 8. Adult immunization standards. Centers for Disease Control and Prevention. Accessed March 15, 2025. https://www.cdc.gov/vaccines-adults/hcp/imz-standards/index.html